Calibration Examples
![]() Calibrating Unstable Substances
Calibrating Unstable Substances
If you want to calibrate unstable substances, the concentration in the samples that are analyzed later may be considerably lower than the concentration in those samples that are analyzed first although originally the concentration was the same. The instability of the substance makes calibrating more difficult. Chromeleon provides two possible solutions:
Sample List
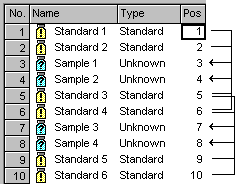
To consider the instability of substances one or several standard samples are added to a series of unknown samples every now and then. The sequence will then appear as follows, for example:
QNT Method/General Tab
The bracketed calibration illustrated in the above figure has been achieved using the Bracketed Calibration Mode (set in the Global Calibration Settings section). The four less decayed standards 1-4 (from positions 1, 2, 5, and 6) are used for calibrating the less decayed unknown samples (samples 1 and 2 from positions 3 and 4). The more decayed standards 3-6 (from positions 5, 6, 9, and 10) are used to calibrate the higher decayed samples 3 and 4 from positions 7 and 8. The calibration curve shows the corresponding Calibration Points, only.
For more information about the Bracketed mode see ![]() Calibration Mode: Bracketed.
Calibration Mode: Bracketed.
![]() Tip:
Tip:
Knowing the half-life of an unstable substance (this is especially true for radioactive substances) is a clever way to calculate the chromatogram, as it would be without the decay. Use a virtual channel (Virtual Channel Driver) to record all chromatograms in this special channel as if the substance would not decay. For a program example, refer to Practical Tips for Device Control ![]() Program Examples for Virtual Channels. Having recorded these Virtual Signals, perform the calibration as described in the above examples for stable substances.
Program Examples for Virtual Channels. Having recorded these Virtual Signals, perform the calibration as described in the above examples for stable substances.
For an overview of the different calibration possibilities provided by Chromeleon, refer to How to …: ![]() Calibrating.
Calibrating.