Standard Addition
Standard Addition is used as a calibration method, mainly in ion and gas chromatography. Standard Addition considers matrix effects during the analysis.
Before the analysis, a known amount of one or more substances is added to a known volume of an unknown sample. In this way, the concentrations of these substances are increased by values that are exactly known. Afterward, the original and the spiked sample are analyzed. For more reliable results, you can spike the unknown sample either several times or with various known quantities.
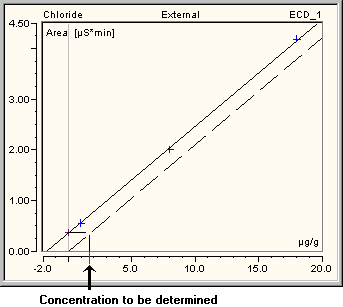
For analysis, the area is plotted against the concentration. (In gas chromatography, the area is plotted against the amount.)

The unknown concentration (amount) of the originally unspiked sample (sample type = Unspiked) is set to zero, i.e., the y-axis is exactly on the concentration of the unspiked sample For evaluation purposes, the calibration line (calibration curve) is shifted parallel by setting the offset to zero. The concentration of the unspiked sample is determined as shown in the picture.
![]() Caution:
Caution:
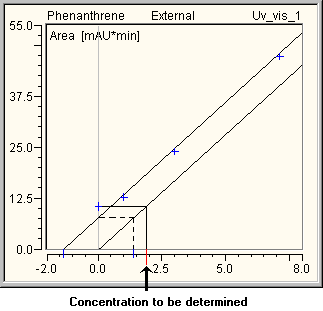
Only if the calibration curve is forced through the origin (i.e., in this case, through the calibration point of the original, unspiked sample), the amount of this sample corresponds to the negative intercept on the x-axis, i.e., to the average of all values based on this sample. For all other calibration types, the calculated amount of the original, unspiked sample may deviate from the negative intercept on the x-axis of the calibration curve.
The picture below illustrates this for the case in which the measured value of the original sample (evaluation: solid line) above the curve, i.e., the corresponding amount, is larger than the negative intercept on the x-axis (broken line):
For more information, refer to Calibrating ![]() Standard Addition.
Standard Addition.