Fluorescence
The immediate emission of light after excitation with light energy is referred to as fluorescence. Fluorescence is often used as the detection principle in HPLC applications. The wavelength of the emitted light is longer than the excitation wavelength. In solutions, the fluorescence spectrum is independent of the excitation wavelength.
The Molecular Orbital (MO) theory provides a more exact explanation:
Molecules are excited by the absorption of light. Absorption arises from the ground state, the lowest vibrational level of the lowest singlet state (S0) and terminates in different excited vibrational levels of the next higher singlet state (S1).
![]()
where:
h = Plank's constant
![]()
where:
c = speed of light
![]() = wavelength
= wavelength
Higher vibrational levels of S1 undergo radiationless transitions to the lowest vibrational level of S1. Finally, fluorescence originates in the lowest vibrational level of S1 and terminates in different excited vibrational levels of S0:
![]()
Also, refer to the image below for clarification:
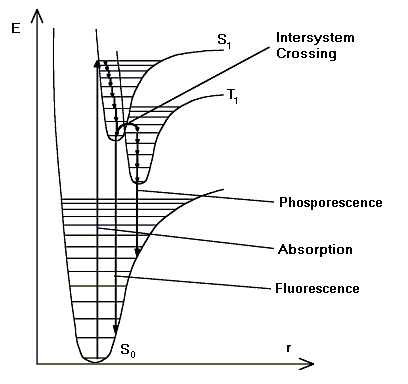
![]() Note:
Note:
Due to the fast relaxation in solution, the emission spectra of electronically excited molecules in solution is independent of the excitation wavelength (also for excitation of, e.g., S0®S2), according to the Kasha rule.